Ions
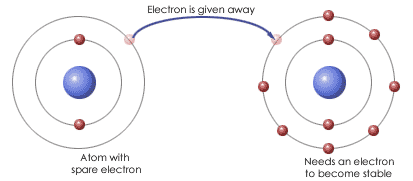
When an atom gains or loses an electron, it becomes an ion.
Atom
Loses electron Gains electron
Anion Cation
Metals Non-metals
Atoms form ions so as to obtain a full valence shell and to become stable. Ions are formed by ionic bonding and covalent bonding. Ionic bonding involves the transfer of electrons,
while covalent bonding involves the sharing of electrons.
My Reflections for Lessons 1, 2 & 3: This topic is an averagely difficult topic with regards to how well an individual is able to memorize and apply the various 'rules' of the drawing of the model. I did not do very well in this topic as I kept making mistakes when drawing the models. At times, counting the number of protons or electrons or neutrons became a problem as I had difficulty remembering how to use the atomic number and nucleon number to calculate the number of protons, electrons and neutrons. Ionic bonding and Covalent bonding are pretty hard to differentiate at times and I would mistake one for the other sometimes. I learnt to remember the hard way when this cost me 4 marks in the term 1 test. I feel that this should be one of my greater focuses as I generally dislike topics related to memorization and calculation.

No comments:
Post a Comment